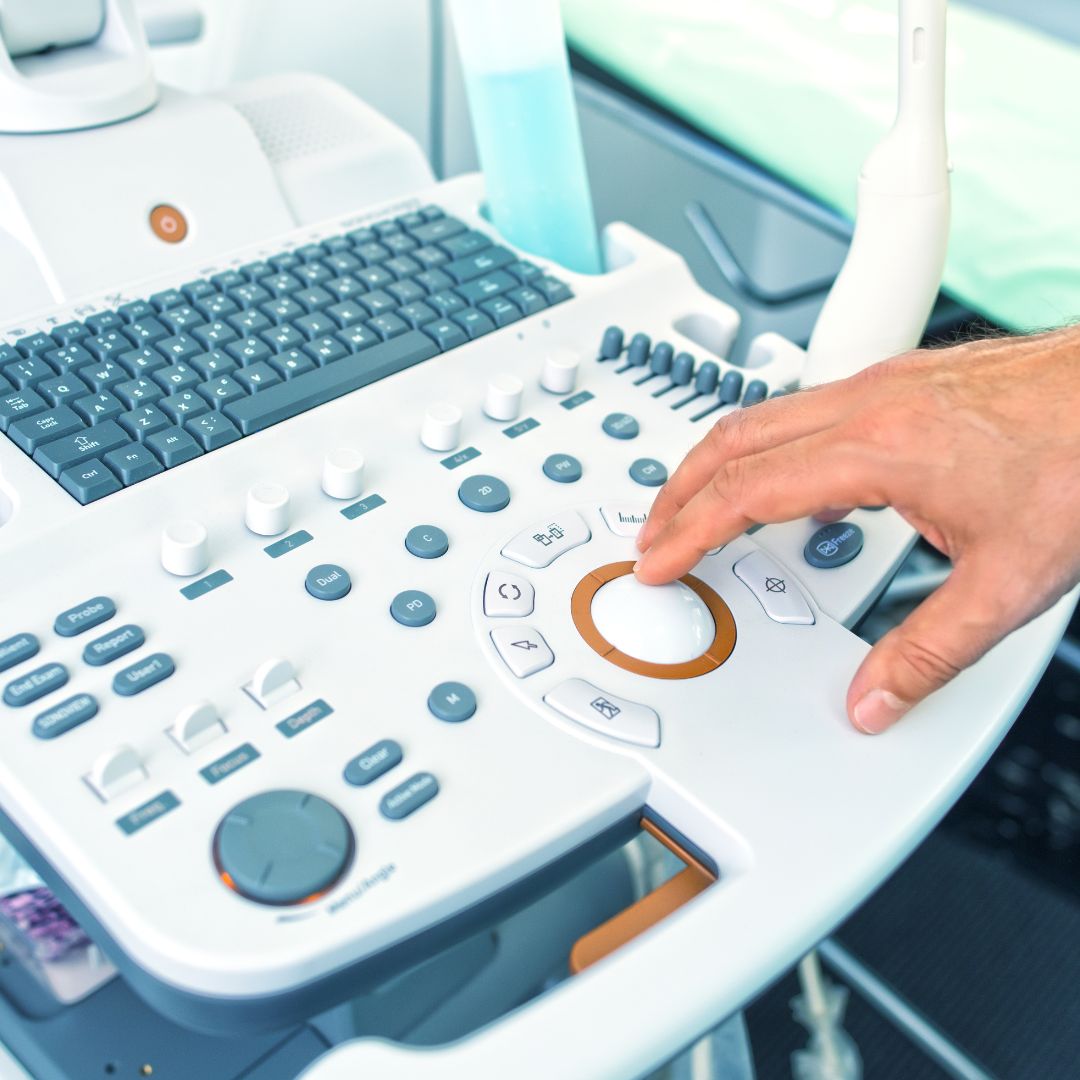
INK FOR MEDICAL GRADE SILICONE APPLICATIONS
The Natron SE-F Series is a 2-component medical-grade silicone ink formulated for marking silicone parts that operate in demanding healthcare and advanced science applications. These medical grade silicone inks meet and exceed the quality standards set by the healthcare, space, and electronics industries. To formulate the SE-F Series we use the highest-purity materials and standards possible. We document each and every raw material from start to finish. This makes it possible to trace every batch produced. Additionally, we produce certificate of compliance for each batch.
Once cured, these non-corrosive, non-toxic silicone inks retain their color and physical properties at low and elevated temperature environments. These inks are also stable and stick well to silicone rubber substrates for long-term use in medical devices and healthcare surfaces.
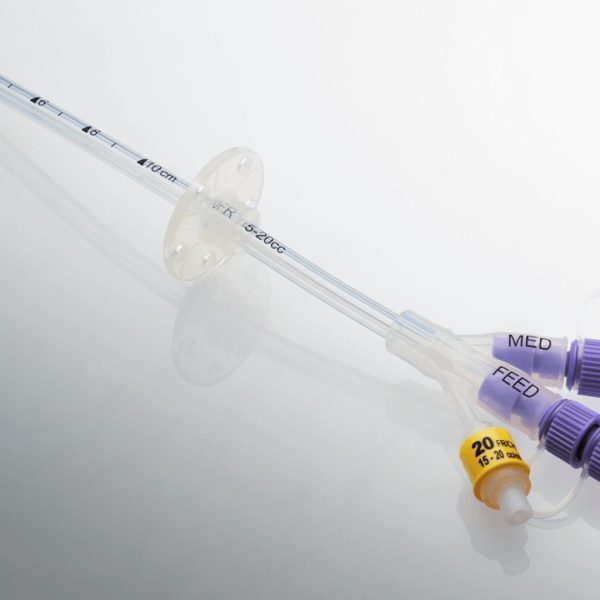
SE-F Series is formulated for pad printing, silk screen marking, and spray coating on silicone components and devices. Examples of these applications include silicone tubing, calendar sheeting, LSR-molded medical parts, etc. Like all Natron printing inks, the SE-F is also designed with ease of use and sustainability in mind. This ink uses less material during application due to its high opacity and lasts longer than any other ink on the market today due to the purity of the materials used.
Medical-grade silicone ink features
Our Natron SE-F ink line has twenty-two high-opacity colors, double any other medical ink available in the market today. In addition, we offer any custom color matching you can think of using all major color matching systems and standards in the world, including AMS-STD-595.
To dry this ink, use heat. This feature makes sure the ink bonds to the substrate, giving it a long service life.
This silicone ink has medical Class VI certification and meets the requirements of the GLP Class VI Test (USP). It also meets the current FDA 21 CFR, Part 58 GLP technical requirements.